Medical Devices 93/42/EEC (MDD), Directive on Active Implantable Medical Devices 90/385/EEC (AIMDD), and Directive on In vitro Diagnostic Medical Devices 98/79/EC (IVDD). As notified body for medical devices, our identification number is 0123. We are able to provide you with the legally required. Whereas medical devices should, as a general rule, bear the CE mark to indicate their conformity with the provisions of this Directive to enable them to move freely within the Community and to be put into service in accordance with their intended purpose; Whereas, in the fight against AIDS and in the light of the conclusions of the Council.

Medical Device Directive 93/42/EEC MDD Directive.PresentationEZE

STATEMENT TRANSITION FROM THE MDD 93/42/EEC DIRECTIVE TO THE EU REGULATION MDR 2017/745 ON

List of Notified Bodies Under Directive 9342 EEC Medical Devices Medical Device Implant

Scope of Application Medical Device Directive 93/42/EECPresentationEZE
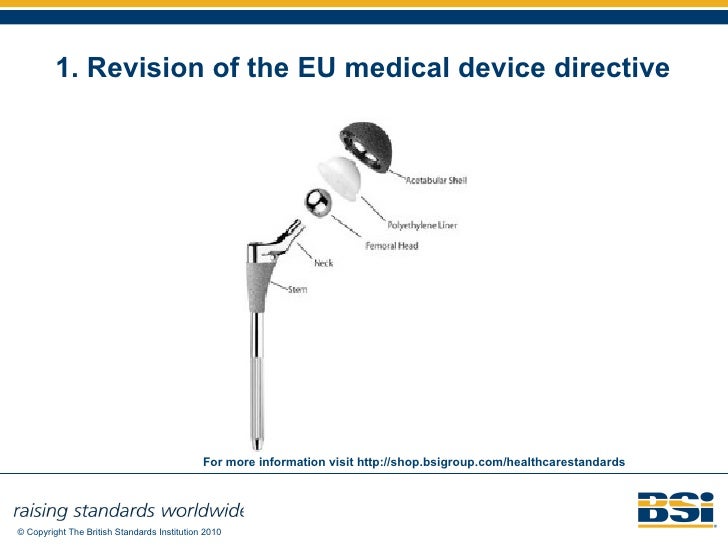
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…
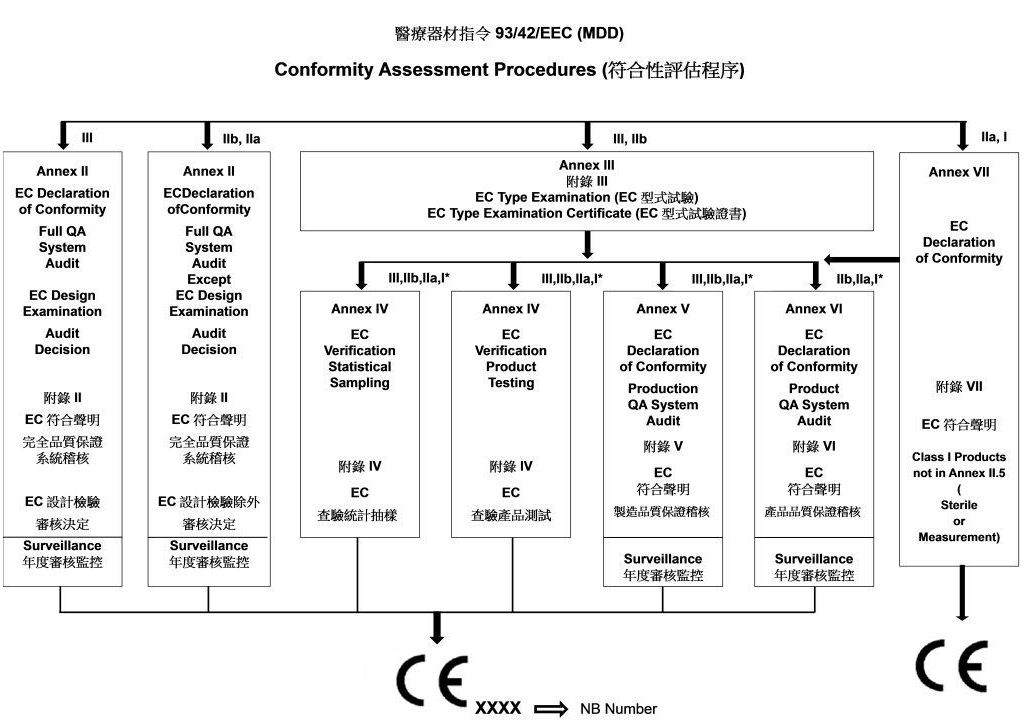
93/42/EEC(MDD)

The Transition From Medical Devices Directive 93/42/EEC To Medical Device Regulation 2017/745 EU

PPT 93/42/EEC Medical Devices Directive 90/385/EEC Active Implantable Medical DevicesDirective

Medical Device Directive 93/42/Eec Certification Service in Sector38, Chandigarh Eucert

DiaTecne
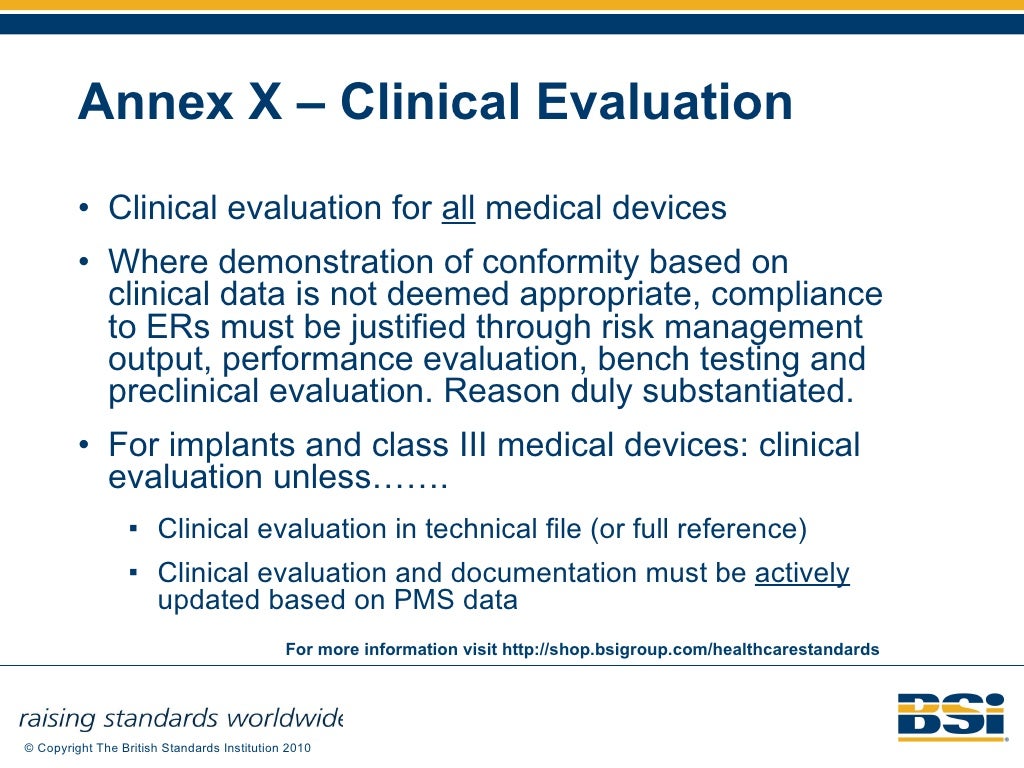
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…

Basic Information about the European Directive 93/42/EEC on

EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…
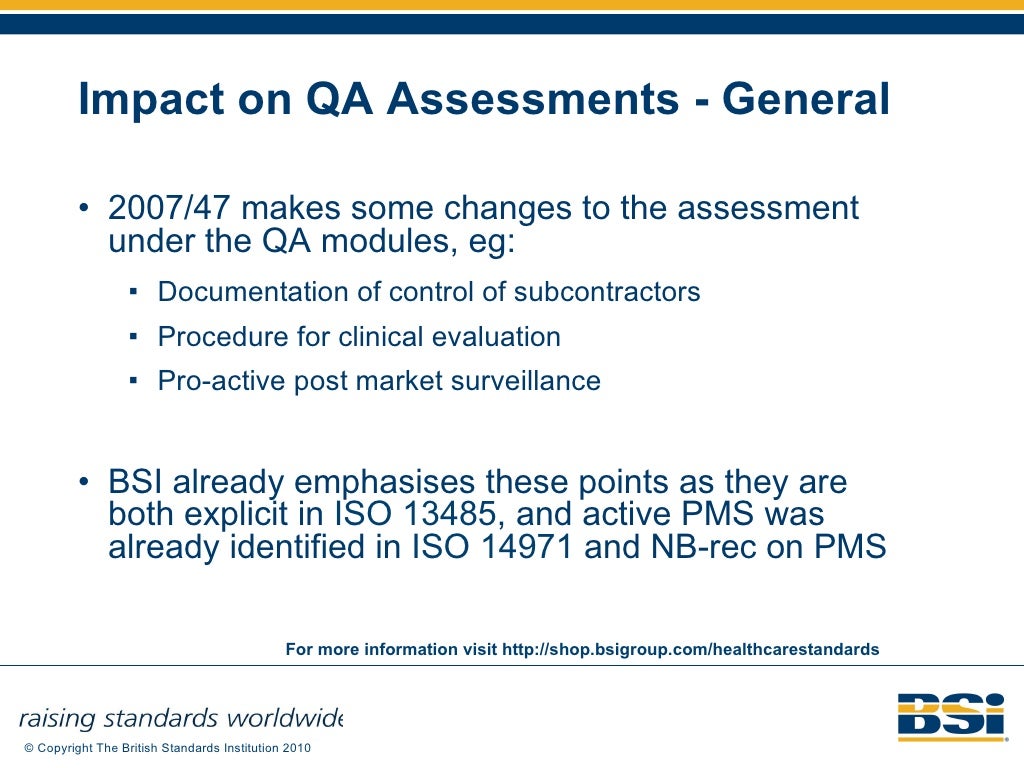
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…
EU Medical Devices Directive M5 Amendment 93 42 EEC Regulatory Update…

MDD 93/42/EEC

医疗器械指令 93/42/EEC?Medical?Devices?Directive(MDD) 口罩等防护产品欧洲CE认证标准汇总 知乎
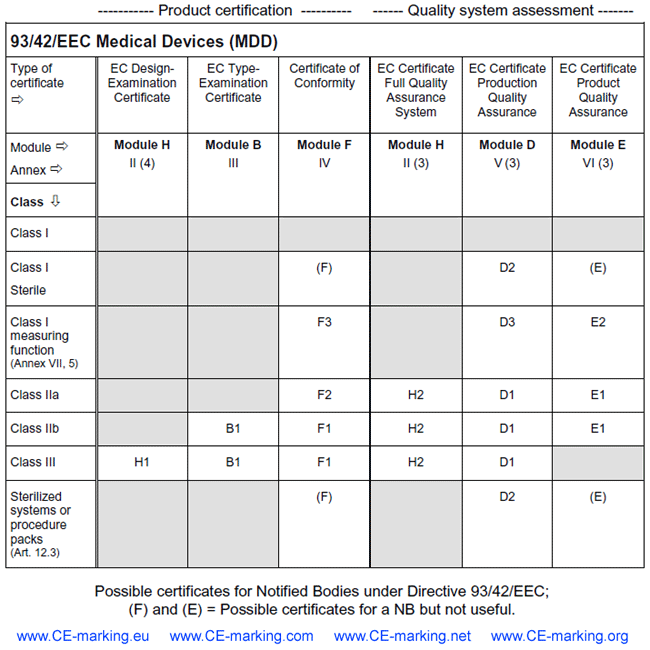
List of all CE marking certificates Notified Bodies can issue under 3 medical devices directives

PPT 93/42/EEC Medical Devices Directive 90/385/EEC Active Implantable Medical DevicesDirective
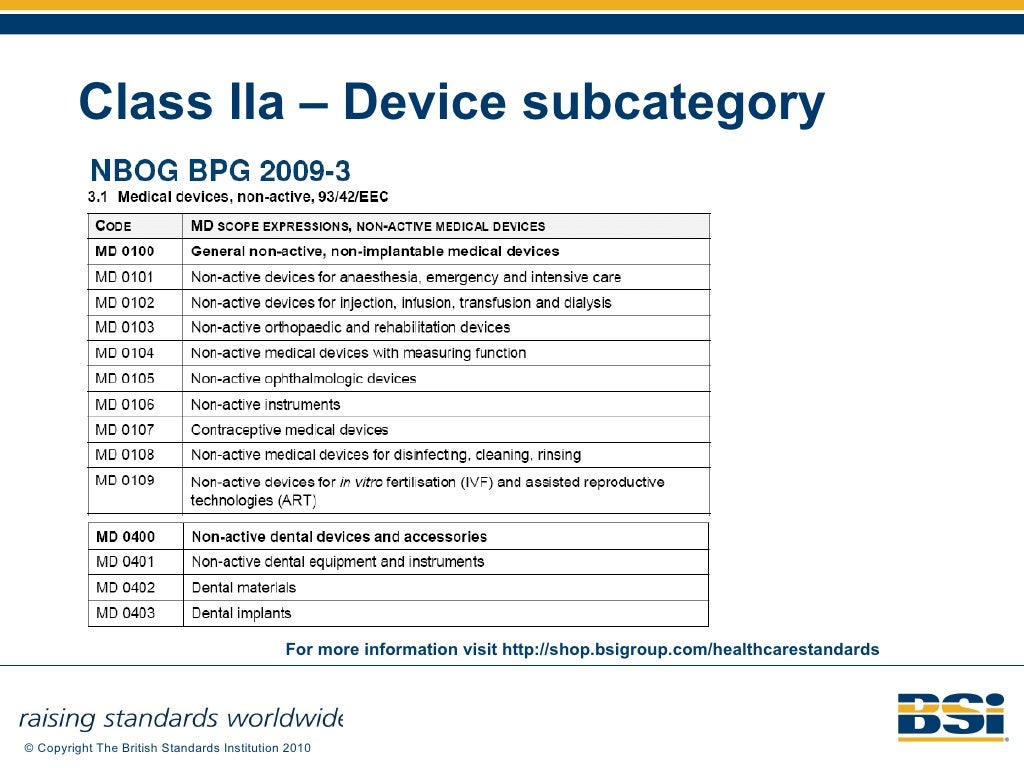
2 Council Directive 93/42/EEC of 14 June 1993 concerning medical devices Document Generated: 2023-09-05 Status: EU Directives are being published on this site to aid cross referencing from UK legislation. After IP completion day (31 December 2020 11pm) no further amendments will be applied to this version.. Medical Device Directive 93/42/EEC. The Medical Device Directive 93/42/EEC defines a "Medical Device" as any instrument, apparatus, appliance, material or other article, whether used. alone or in combination, including the software necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: